Medical Device Integration
HOME – MEDICAL DEVICE INTEGRATION
Seamless Connectivity with 500+ Medical Devices
Our Medical Device Integration solutions establish certified interfaces with over 500 medical devices and equipment from leading manufacturers worldwide, creating a truly connected healthcare ecosystem. By automatically capturing data from laboratory analyzers, imaging equipment, patient monitors, ventilators, infusion pumps, and anesthesia machines directly into electronic health records, we eliminate manual transcription errors, reduce documentation burden on clinical staff, and ensure that critical patient data flows seamlessly from device to medical record in real-time.
The integration platform supports bidirectional communication—not only capturing results and measurements from devices but also transmitting orders and parameters from hospital information systems to equipment. This closed-loop integration enables features like barcode-verified medication administration with smart pump integration, automatic ventilator setting adjustments based on protocols, and laboratory order transmission directly to analyzers, creating workflows that are safer, more efficient, and less prone to human error.
Designed for healthcare facilities seeking to maximize their technology investments while improving patient safety and operational efficiency, our device integration solutions work across all clinical environments—from intensive care units and operating rooms to general medical-surgical floors, emergency departments, and outpatient settings. Whether you’re a community hospital with basic monitoring equipment or an academic medical center with cutting-edge specialized devices, our flexible integration architecture adapts to your specific equipment portfolio and clinical workflows.
- Laboratory Analyzers & Instruments
Certified interfaces with chemistry analyzers, hematology systems, coagulation analyzers, immunoassay platforms, blood gas analyzers, urinalysis systems, and microbiology instruments from manufacturers including Roche, Abbott, Siemens, Beckman Coulter, Ortho Clinical Diagnostics, bioMérieux, and BD. Bidirectional communication enables electronic order transmission to instruments and automatic result upload to LIS and EMR, eliminating manual entry and accelerating turnaround times while ensuring accuracy.
- Patient Monitoring Systems
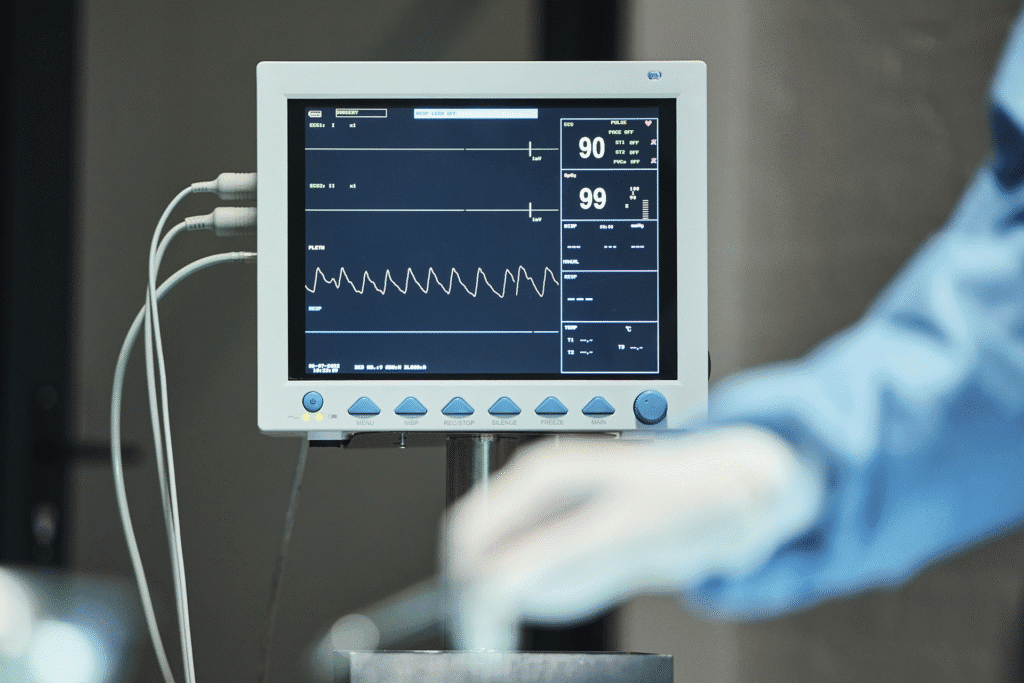
Real-time vital signs capture from bedside monitors, telemetry systems, and central station monitoring including Philips IntelliVue, GE Healthcare CARESCAPE, Nihon Kohden, Mindray, and Masimo devices. Automatic documentation of heart rate, blood pressure, respiratory rate, oxygen saturation, temperature, and waveform data flows directly into nursing flowsheets, reducing documentation time and ensuring accurate trending. Configurable alarm integration enables critical value notifications through hospital communication systems.
- Imaging & Radiology Equipment
DICOM-compliant integration with X-ray, CT, MRI, ultrasound, mammography, nuclear medicine, and fluoroscopy equipment from all major manufacturers. Automatic study routing from modality to PACS ensures images are immediately available for radiologist interpretation. Modality worklists transmitted from RIS to imaging devices improve workflow efficiency and reduce registration errors. Dose reporting interfaces support radiation safety monitoring and regulatory compliance.
- Critical Care & Life Support Devices
Integration with mechanical ventilators (Dräger, Hamilton, GE, Medtronic), infusion pumps (Baxter, B. Braun, Smiths Medical, ICU Medical), anesthesia machines (GE, Dräger, Mindray), and dialysis systems. Automatic capture of ventilator settings, respiratory parameters, infusion rates, medication administrations, and dialysis treatment data. Smart pump integration with medication library verification reduces medication errors. Anesthesia record automation saves time while improving documentation completeness.
- Point-of-Care Testing Devices
Connectivity with glucose meters, blood gas analyzers, coagulation monitors, cardiac marker systems, urinalysis devices, and other point-of-care instruments used at bedside, in emergency departments, and operating rooms. Results flow automatically to laboratory information systems and electronic health records with full traceability including operator identification, quality control verification, and timestamp documentation. Supports regulatory compliance for CLIA-waived and moderate complexity testing.
- Specialized & Departmental Equipment
Interfaces with cardiology devices (ECG machines, stress test systems, Holter monitors, echocardiography), respiratory therapy equipment (pulmonary function systems, sleep study devices), physical therapy measurement tools, and other specialty equipment. Custom integration development available for unique or proprietary devices specific to your institution’s needs. Our engineering team has experience creating interfaces for virtually any medical device capable of electronic communication.
Ready to Create a Fully Connected Care Environment?
Discover how our medical device integration solutions can eliminate manual data entry, reduce errors, and free your clinical staff to focus on patient care rather than documentation. Schedule a consultation to assess your integration opportunities.
- FAQs
Frequently Asked Questions About Medical Device Integration
What if we have a device that's not on your standard interface list?
Our integration engineering team can develop custom interfaces for virtually any medical device capable of electronic data output. We'll work with the device manufacturer and your team to understand communication protocols and develop a certified interface that meets your workflow requirements and regulatory standards.
How do you ensure data accuracy when capturing information from devices?
All interfaces include validation rules that verify data completeness and reasonability before committing to medical records. Any questionable values trigger alerts for clinical verification. We maintain detailed audit trails showing data source, capture time, and any transformations applied. Regular quality checks compare device-captured data against manual entries to validate interface accuracy.
Can nurses override or edit device-captured data if needed?
Yes, clinicians retain full control over documented data. Device-captured information can be reviewed and edited if clinical judgment indicates the automated reading doesn't reflect the patient's actual condition. All edits are documented with reason codes and maintain the original device value for comparison, creating a complete audit trail.
What happens if the interface connection fails or a device goes offline?
The system includes robust error handling and buffering capabilities. If connection is temporarily lost, devices typically store data locally until connection is restored, then transmit automatically. Staff receive notifications of interface failures so manual documentation can resume if necessary. Our monitoring systems alert IT teams immediately when interfaces stop functioning for rapid resolution.
How does device integration improve patient safety?
Automatic data capture eliminates transcription errors that occur with manual entry. Real-time data availability enables faster clinical response to changes in patient condition. Closed-loop medication administration with smart pump integration verifies the right drug, dose, route, and patient before infusion begins. Trending and alerting based on device data can identify deteriorating patients earlier than intermittent manual assessments.
What's involved in implementing device integration in our facility?
Implementation includes device inventory assessment, interface configuration, network connectivity verification, workflow analysis, staff training, and validation testing before go-live. Timeline varies based on number and complexity of devices but typically ranges from 2-4 months. We coordinate with device manufacturers and your IT team to minimize disruption and ensure successful deployment.